The RIH Foundation is passionate about supporting advancements in research and innovation because we believe it is essential for delivering exceptional patient care and driving medical progress. By fostering a culture of innovation, we not only improve health outcomes for our community but also create an environment where the brightest minds in medicine are inspired to work. Investing in research and innovation ensures that we remain at the forefront of medical excellence, offering our community the best care possible.
Innovation Highlights
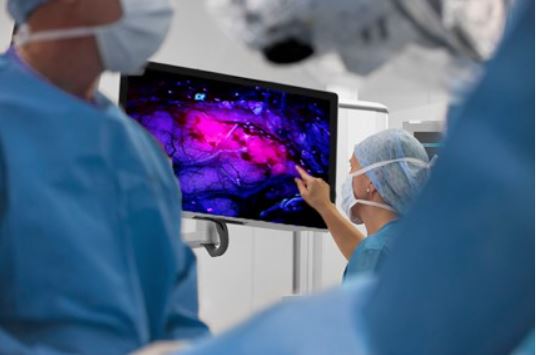
Shining a Light on Precision: How RIH Neurosurgeons Are Using Gleolan to Revolutionize Local Brain Tumour Surgery
Research Highlights
“Supporting clinical research at RIH is very important for attracting and keeping medical professionals. By building a strong research environment at RIH, we not only improve evidence-based medical knowledge and patient care, but we will also attract top research talent to Kamloops as many specialists want to do innovative and impactful research in addition to their medical practice. Investing in clinical research at RIH also gives patients access to the latest technologies and treatments and helps to create local solutions to healthcare issues. The RIH Foundation’s commitment to supporting research will help to provide opportunities for professional growth which keeps medical professionals motivated and engaged, leading to better patient outcomes and a stronger healthcare system for families in Kamloops.” – Dr. Barbara Jean Buckley, Manager, Research Compliance | Researcher | Adjunct Professor Family Practice Residency Program



MYCOVACC: Myocarditis and/or pericarditis following mRNA COVID-19 VACCination national surveillance study (MYCOVACC)
There are many causes of myocarditis and/or pericarditis. Rare reports of myocarditis and/or pericarditis have occurred after COVID-19 infection or after receiving a mRNA COVID-19 vaccination. Canadian and international data point to a slightly higher rate of myocarditis and/or pericarditis than would normally be expected in the general population. The purpose of this study is to collect information on how all individuals given a myocarditis and/or pericarditis diagnosis in Canada are identified, treatment, and outcomes over the study duration. This data may help to contribute to a better understanding of this medical condition broadly. Dr. Steven Sra is the Qualified Investigator supporting Interior Health’s contribution to this study.
Sponsor: Centre for Cardiovascular Innovation, The Canadian Cardiovascular Society (CCS) is co-sponsoring this study. Funded By: Public Health Agency Canada (PHAC)
The DAL-302 (dal-GenE-2) trial
This study is to evaluate the effect of the drug dalcetrapib in patients with the AA genetic variant who have had a recent Acute Coronary Syndrome (ACS) event to see if dalcetrapib can reduce the risk for another ACS event.
Dal-GenE-2 is a North American, muti-centered study with the goal of approximately 14,000 participants being screened and 2,000 participants randomized. Dal-GenE-2 is the most recent cardiovascular study supported by the Qualified Investigator at RIH, Dr. Steven Sra.
Sponsor: DalCor Pharmaceuticals Canada Inc. (DalCor)

REFINE-ICD: Risk Estimation Following Infarction, Non-Invasive Evaluation ICD efficacy (REFINE ICD)
The REFINE ICD study tests whether an implantable cardioverter defibrillator (ICD) can increase the likelihood of survival in patients at risk of heart irregularities. Dr. Steven Sra and the Clinical Research team at RIH have supported 26 local participants in the REFINE-ICD study since 2019.
Sponsor: University of Calgary

Aldosterone Blockade for Health Improvement Evaluation in End Stage Renal Disease (ACHIEVE)
This study is examining whether spironolactone, a medication used for heart problems, can also improve health outcomes for patients with severe kidney disease who are on dialysis.
The study aims to determine if spironolactone can reduce heart-related complications and death rates in these patients. This global study will involve up to 2,750 participants from 200 centers worldwide, with a goal of having 25 people from the Kamloops region participate. Dr. Joslyn Conley is the qualified investigator lead for this study.
Sponsor: Population Health Research Institute (PHRI)

Phenobarbital for Acute Alcohol Withdrawal: A Two-Year Retrospective Study (Phase 1)
Dr. David Jerome (ER Physician) and study team are performing a two-year retrospective chart review to assess the efficacy of phenobarbital with or without benzodiazepines for treatment and management of acute alcohol withdrawal in the RIH-ED.
They hope to determine if the use of phenobarbital alone or with adjunct benzodiazepines improves clinical outcomes for those with acute alcohol withdrawal symptoms compared to the current standard of care (benzodiazepines alone).
A planned future study (Phase II) will go on to explore the use of phenobarbital for acute alcohol withdrawal symptoms to see if it will result in better patient outcomes, shorter lengths of stay, less use of specialized resources, and fewer emergency department re-visits within 72 hours of discharge.
Long-COVID: Building a Model of Community Support for Post-Acute Sequelae of COVID-19 Patients within Interior Health: Research, Evaluation, and Care in a Learning Health System
The first phase of this research explored the impact of providing IH based Long-COVID patients and primary care providers access to a virtual Post-COVID community-based interdisciplinary care team. This Long-COVID interdisciplinary team led by Dr. Barbara Jean Buckley (IH Research) provided support, online classes, resources, education, and opportunities for patients and providers to participate in Long-COVID research.
Phase II of this project has taken what was learned from patients in the first phase of the study to now focus the research on ways to advance patient care by helping to better support primary healthcare providers who care for patients living with Long-COVID in the Interior Health community. In this phase we are actively seeking input from primary care providers in order to better understand their needs. We will be using current research to create innovative and targeted practice supports and resources for primary care physicians to help them provide evidence-based care for their patients living with long-COVID.

Hip Attack-2: Hip Fracture Accelerated Surgical Treatment and Care Track – 2
This is a multi-centre, international trial to determine whether accelerated surgery for hip fracture in patients with acute myocardial injury is superior to current standard care at reducing mortality rates in the 90 days after the hip fracture occurred.
This study is being reviewed and supported by Dr. Jonathan Bourget Murray, RIH orthopedic surgeon.
Sponsor: Public Health Research Institute

EASi-KIDNEY: Aldosterone Synthase Inhibition & Empagliflozin in CKD
This drug trial will test whether a new drug called BI 690517 can slow the progression of kidney disease, reduce hospitalizations for heart failure, and lower death rates from heart problems in people with chronic kidney disease. This multisite study will involve about 11,000 participants.
Recruitment for this trial will begin in 2024, and results are expected by 2028. The trial is organized by the Renal Studies Group, and Dr. Conley at RIH will lead as the qualified investigator at RIH.
Sponsor: Boehringer Ingelheim